Learn more, reach out. Click to contact your sales representative.

RECELL® Spray-On SkinTM Cells in Real Life
Case Studies in Success


SEE THE BENEFITS
FOR YOURSELF
Expedite the healing process with early point-of-care treatment using RECELL1

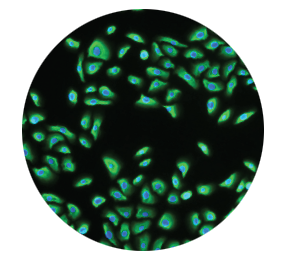
Keratinocytes regenerate
the epidermis2,3
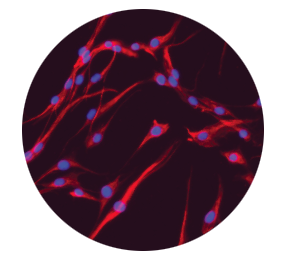
Dermal fibroblasts deposit new extracellular matrix proteins2
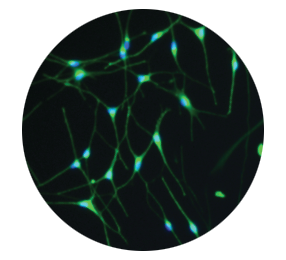
Melanocytes produce melanin to allow restoration of natural pigmentation3
Limb Salvage Clinical Case
Calcaneal Wound
Effective treatment of a 10-month-old peripheral vascular disease wound and osteomyelitis to left calcaneus using RECELL Spray-On Skin™ Cells and a 1.5:1 meshed split-thickness skin graft (mSTSG)
81-year-old male
Comorbidities:
Critical limb ischemia, insulin dependent diabetes mellitus, peripheral neuropathy, hypertension, chronic kidney disease, atrial fibrillation, pacemaker, chronic anemia
Total treatment area:
15 cm2
Prior treatment:
Debridement, medications
for osteomyelitis, hyperbaric oxygen therapy, negative pressure wound therapy
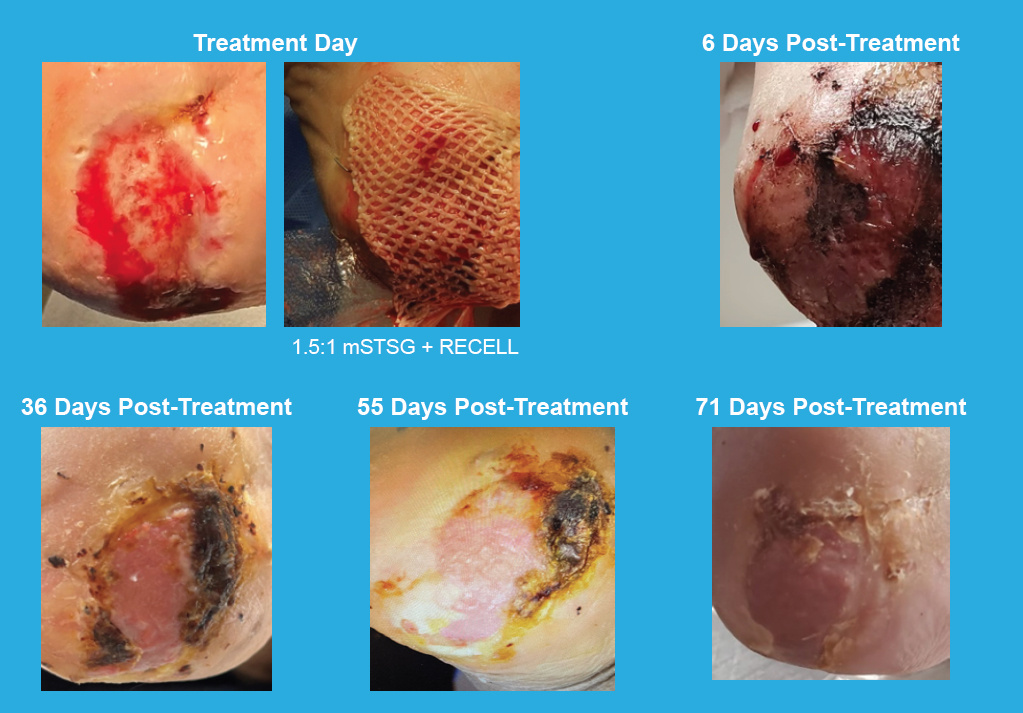
Photos courtesy of Shital Pema, DPM (Orthopedic Associates of Dayton, Inc.—Dayton, OH)
- Instructions for Use. RECELL® Autologous Cell Harvesting Device.
- Freedberg et al. J Invest Dermatol. 2001;116:633-640.
- Hirobe. Dermatol Sin. 2014;32(4):200-204.
INDICATIONS FOR USE: The RECELL Autologous Cell Harvesting Device and RECELL GO Autologous Cell Harvesting Device are indicated for the treatment of thermal burn wounds and full-thickness skin defects. The RECELL Device and RECELL GO Device is used by an appropriately licensed and trained healthcare professional at the patient’s point of care to prepare autologous Spray-On Skin Cells for direct application to acute partial-thickness thermal burn wounds in patients 18 years of age and older or application in combination with meshed autografting for acute full-thickness thermal burn wounds in pediatric and adult patients and full-thickness skin defects after traumatic avulsion (e.g., degloving) or surgical excision (e.g., necrotizing soft tissue infection) or resection (e.g., skin cancer) in patients 15 years of age and older.
CONTRAINDICATIONS: RECELL and RECELL GO are contraindicated for the treatment of: wounds clinically diagnosed as infected or with necrotic tissue present in the wound bed, patients with a known hypersensitivity to trypsin or compound sodium lactate (Hartmann’s) solution, and patients with a known hypersensitivity to anesthetics, adrenaline/epinephrine, povidone-iodine, or chlorhexidine solutions.
WARNINGS: Autologous use only. Control infections and excise all necrotic tissue on the wound bed prior to application of the meshed autograft and/or Spray-On Skin Cells. Wound beds treated with a cytotoxic agent (e.g., silver sulfadiazine) should be rinsed prior to application. Choose a donor site with no evidence of cellulitis or infection and process skin immediately. The enzyme is animal-derived and freedom from infectious agents cannot be guaranteed.
PRECAUTIONS: RECELL and RECELL GO are not intended for use without a meshed autograft for the treatment of acute full-thickness burn wounds or full-thickness skin defects. The safety and effectiveness of the device have not been established for the treatment of: full-thickness skin defects on the hands and genitalia; full-thickness burn wounds in patients younger than 28 days of age; and partial- thickness burn wounds >320 cm2, on the hands and articulating joints, or in patients with wounds totaling >20% TBSA.
INSTRUCTIONS FOR USE: Consult the Instructions for Use at avitamedical.com for complete Safety Information prior to using RECELL and RECELL GO.
