RECELLerate healing. Call today for your in-person demonstration
800-567-9076.

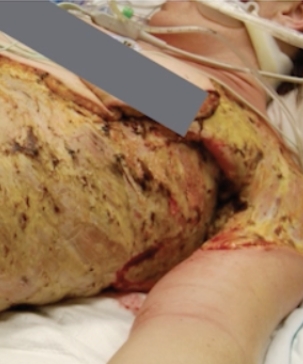
Use of RECELL in combination with meshed STSG in the treatment of wounds caused by necrotizing fasciitis.
Kevin N Foster, MD, MBA, FACS from the Arizona Burn Center, Phoenix, AZ shares a case of a 37-year-old female with necrotizing fascitis that was excised, and the resulting wounds were treated with RECELL. Two weeks post-treatment, her wounds were 95% re-epithelialized and remained healed one month later.
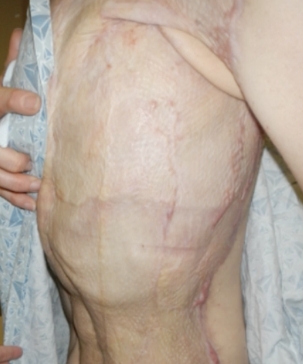
SEE THE FULL STORY AND HER 52-WEEK OUTCOME.
SEE THE FULL STORY AND HER 52-WEEK OUTCOME.
Wound types
Use Spray-On Skin Cells:
• In conjunction with split-thickness skin graft (STSG) for treatment of full-thickness skin defects after:
– Traumatic avulsion (eg, degloving, abrasion)
– Surgical excision (eg, fasciotomy, necrotizing fasciitis)
– Surgical resection (eg, cancer removal)
– Thermal injury (e.g. burn)
• Alone for the treatment of deep partial-thickness wounds after:
– Thermal injury (e.g. burn, road rash)
Benefits of RECELL versus standard of care
Benefits of RECELL versus standard of care
• Significantly less donor skin needed1
• Fewer procedures for definitive closure1-3
• Reduced pain and scarring at the RECELL-harvested donor site4
• Reduction in length of stay for burns <50% TBSA3,5,6
References
1. Instructions for Use. RECELL® Autologous Cell Harvesting Device.
2. Foster K, Bilir P, Kruger E, et al. Cost-effectiveness of RECELL® Autologous Cell Harvesting Device (ACHD) versus STSG for treatment of severe burns in the United States. Presented at the American Burn Association 2018 Annual Meeting, April 2018.
3. Kowal S, Kruger E, Bilir P, et al. Cost effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther. Published online May 7, 2019. doi:10.1007/s12325-019-00961-2.
4. Holmes JH, Molnar JA, Carter JE, et al. A comparative study of the RECELL® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694-702.
5. Carter JE, Carson JS, Hickerson WL, et al. Length of stay and costs with autologous skin cell suspension versus split-thickness skin grafts: burn care data from US centers. Published online ahead of print, 2022 Sep 14. Adv Ther. 2022;10:1007/s12325-022-02306-y.
6. Carson JS, Carter JE, Hickerson WL, et al. Analysis of real-world length of stay data and costs associated with use of autologous skin cell suspension for the treatment of small burns in U.S. centers [published online ahead of print, 2022 Dec 5]. Burns. 2022;S0305-4179(22)00299-6. doi:10.1016/j.burns.2022.11.007.
Important Safety Information
Indications For Use
The RECELL Autologous Cell Harvesting Device is indicated for the treatment of thermal burn wounds and full-thickness skin defects. The RECELL Device is used by an appropriately licensed and trained healthcare professional at the patient’s point of care to prepare autologous Spray-On Skin Cells for direct application to acute partial-thickness thermal burn wounds in patients 18 years of age and older, or application in combination with meshed autografting for acute full-thickness thermal burn wounds in pediatric and adult patients and full-thickness skin defects after traumatic avulsion (e.g., degloving) or surgical excision (e.g., necrotizing soft tissue infection) or resection (e.g., skin cancer) in patients 15 years of age and older.
Contraindications
The device is contraindicated for the treatment of: wounds clinically diagnosed as infected or with necrotic tissue present in the wound bed, patients with a known hypersensitivity to trypsin or compound sodium lactate (Hartmann’s) solution, and patients with a known hypersensitivity to anesthetics, adrenaline/epinephrine, povidone-iodine, or chlorhexidine solutions.
Warnings
Autologous use only. Control infections and excise all necrotic tissue in the wound bed prior to application of the meshed autograft and/or Spray-on Skin Cells. Wound beds treated with a cytotoxic agent (e.g., silver sulfadiazine) should be rinsed prior to application. Choose a donor site with no evidence of cellulitis or infection and process skin immediately. The enzyme is animal-derived and freedom from infectious agents cannot be guaranteed.
Precautions
The device is not intended for use without a meshed autograft for the treatment of acute full-thickness burn wounds or full-thickness skin defects. The safety and effectiveness of the device have not been established for the treatment of: partial-thickness burn wounds >320 cm2, on the hands and articulating joints, or in patients with wounds totaling >20% TBSA; full-thickness burn wounds on the hands and articulating joints, or in patients younger than 28 days of age; full-thickness skin defects on the hands and genitalia.
Instructions For Use
Consult the Instructions for Use at www.AVITAmedical.com for complete Safety Information prior to using RECELL.

For your in-person
demonstration of RECELL
call 800-567-9076
® 2024 AVITA Medical, Inc.