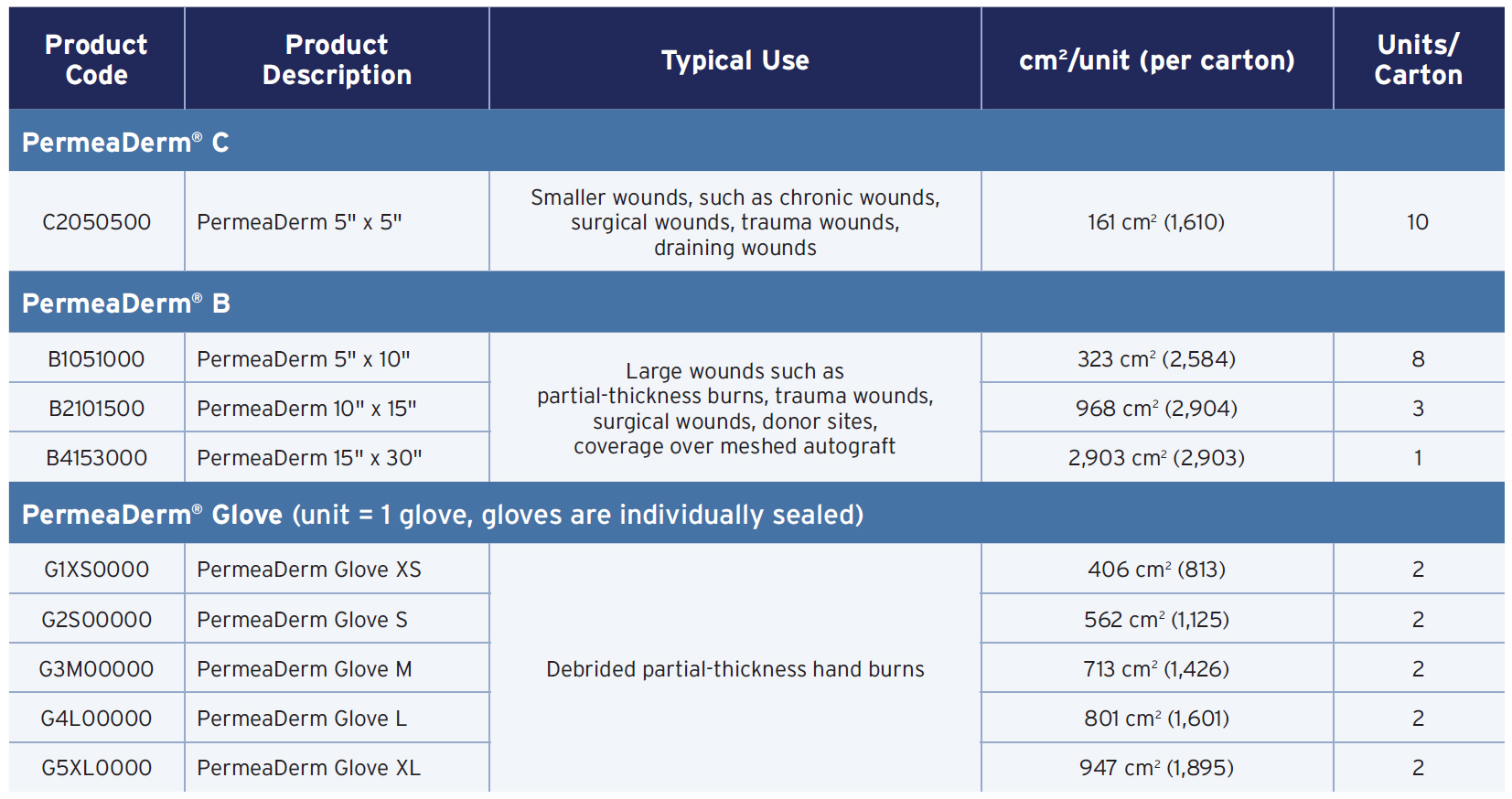
FDA-cleared for partial-thickness wounds, donor sites, and coverage of meshed autografts
See your patient through healing with flexibility and convenience with a proven next-generation technology
Easy to Use
- Simple, one-time application
- Transparency allows at-a-glance wound assessment
- Quickly and intimately adheres to wound surface
- Flexible and adherent even on articulating joints
- Highly water resistant
Convenient and Versatile
- Suitable for wounds, donor sites, and over meshed autografts
- Expanded treatment utility with large sheets (up to 2,903 cm2) and glove options
- Less prone to stick to secondary dressings
- Usable without a secondary dressing after adherence
Proven Effective
- Creates optimal healing environment allowing epidermal migration
- Customizable moisture management (defined porosity through stretch)
- Provides bacterial barrier
- Protects wounds from trauma
Unique, patented, dual layer, biosynthetic wound matrix aids in the function of lost epidermis until re-epithelialization1,2

AVITA Medical, focused on transforming lives through innovative ways of healing skin.
800-567-9076
Fenestrated Outer 2D Silicone Epidermal Analogue facilitates diffusion of O2, CO2, and exudate to/from the wound, without sticking to a secondary dressing
Inner 3D Micro-Support System with biocoating, which expedites adherence to wound bed and supports early mobilization
Case study: Deep Partial-thickness Burn (lower extremity)
12 year-old female • PermeaDerm® Sheet
Healing achieved within 15 days
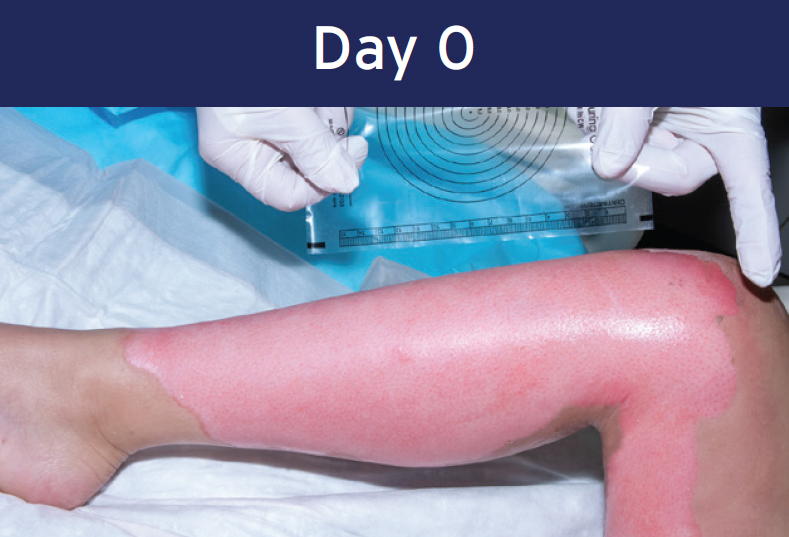
Wound size 516 cm2
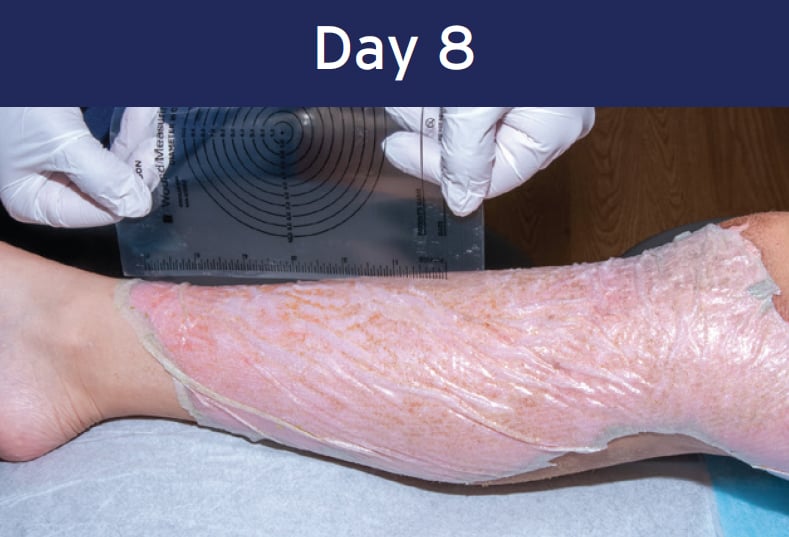
Secondary adherence completed
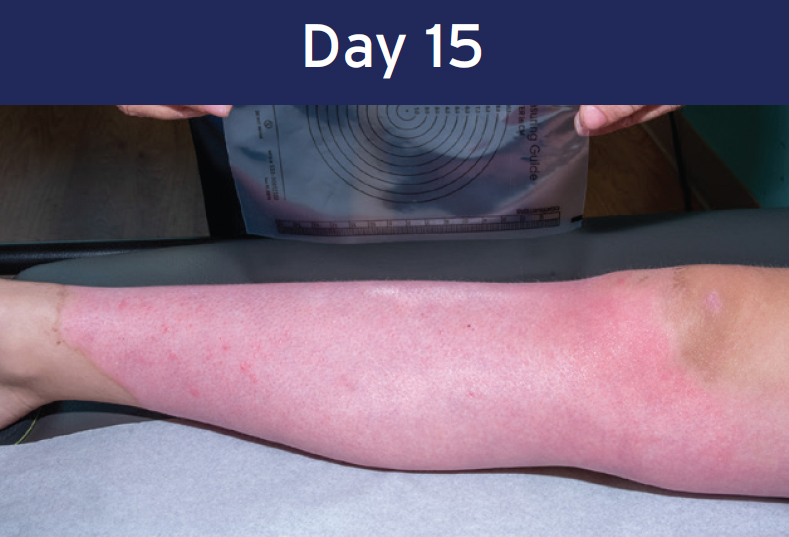
Re-epithelialization completed and wound healed
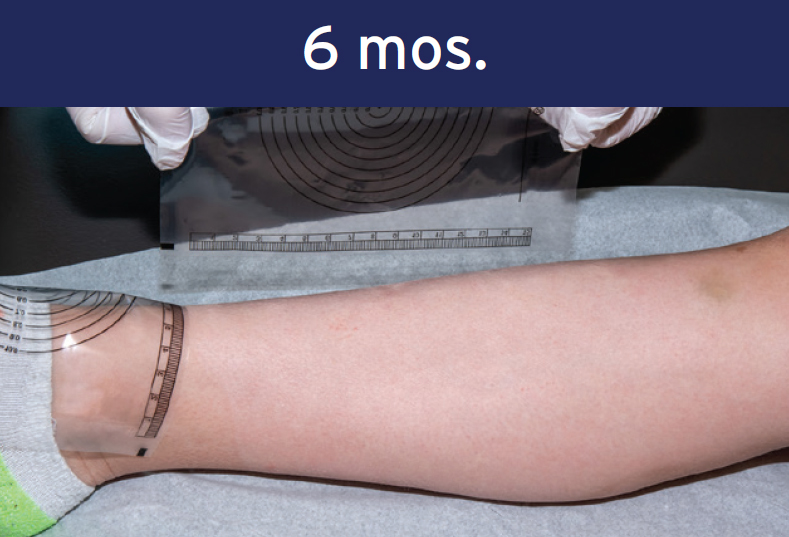
Optimal outcome with no scar
Call today for your in-person demonstration
800-567-9076
2. FDA PermeaDerm 510(k) Premarket Notification.