
Cohealyx™ Collagen Dermal Matrix is bioengineered for full-thickness wound management. Balanced using proprietary TetraPure™ Technology, it supports cellular infiltration and revascularization.1
Built for speed. Designed for confidence.
TetraPure Technology
Uniquely purified and bioengineered with ideal pore structure for integration into the wound bed and revascularization.1,2
Close with Confidence
Revascularization and wound bed readiness evident by day 7 in pre-clinical studies.1
Dependable Performance
Controlled resorption in the wound bed.3
One-of-a-Kind Portfolio
Cohealyx, RECELL®, and PermeaDerm® are exclusive solutions offered by AVITA Medical.
Balanced Collagen Composition
Purified from young bovine dermis, Cohealyx contains Type I and Type III collagen. Type III collagen is linked to reduced scarring and contraction.4-7
The Cohealyx Advantage
Cohealyx’s advanced structure promotes cellular migration and revascularization, creating an optimal wound environment.
Request Information
How Cohealyx Works
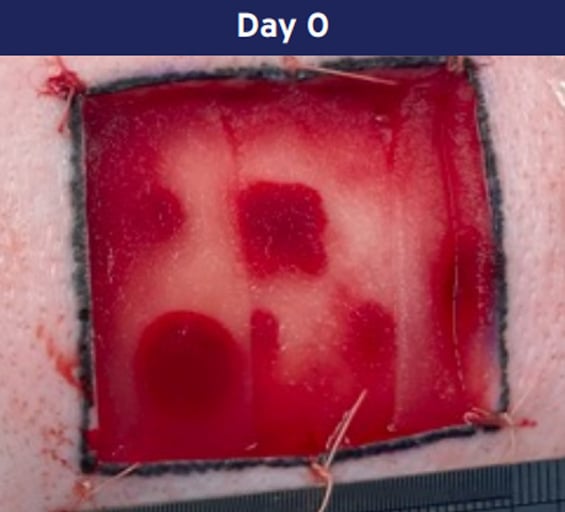
Application:
Cohealyx is placed on a cleanly excised wound bed.
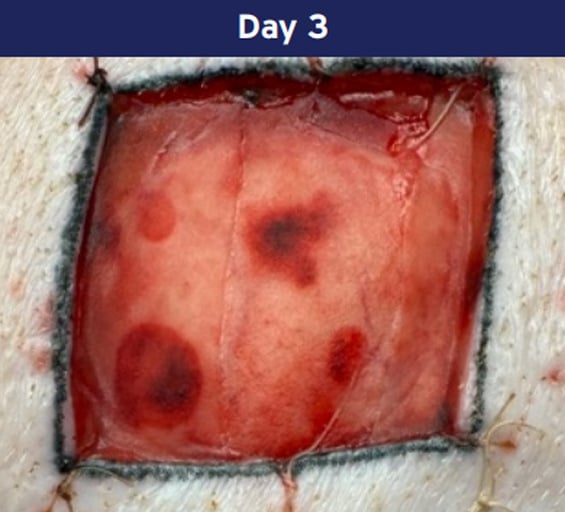
Integration:
Cells migrate into the porous structure and revascularization progresses.
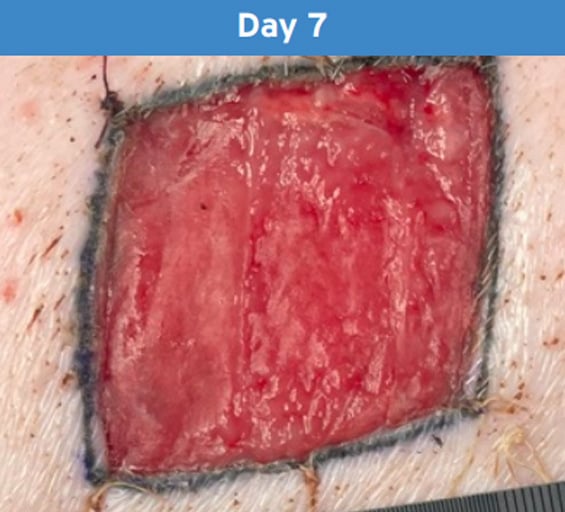
Ready for Closure:
Cohealyx is integrated into the wound bed, supporting definitive closure management.
Photographs from porcine excision wound model. Animal model results do not necessarily translate to clinical results.

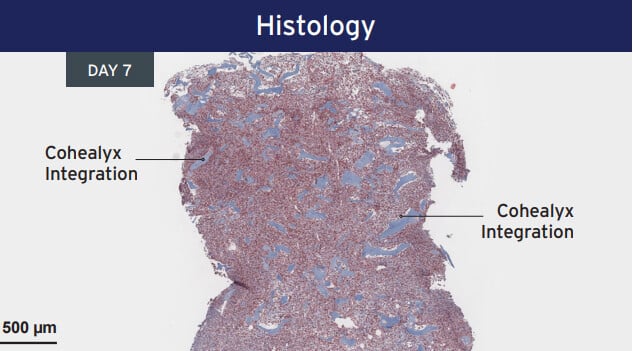
Histology from porcine excision wound model. Animal model results do not necessarily translate to clinical results.
How Cohealyx Excels
| Feature | Coheaylx | Why It Matters |
| Collagen type | Type I & III from young bovine dermis | Type III collagen is linked to reduced scarring and contraction5,6,7 |
| Vascularization time | As early as 7 days in pre-clinical studies | Early closure is linked to better patient outcomes8 |
| Resorption | Controlled resorption: approx. 30 days1,3 | Provides an initial scaffold for cellular ingrowth and revascularization |
| Flexibility | Soft and pliable | Easily conforms to the wound bed |
| Cost effective | Economical with simple, flat pricing | Expands access to advanced wound management |
| Thickness | 3 mm | Provides volumetric build1 |
Experience Cohealyx in Complex Wound Applications
Case Details: 48-year-old-male. Right hand and wrist, 440 cm2.
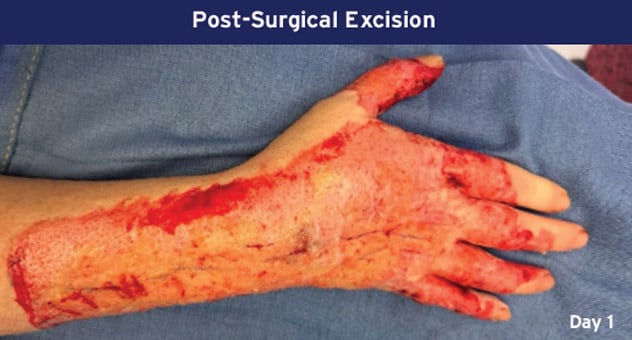
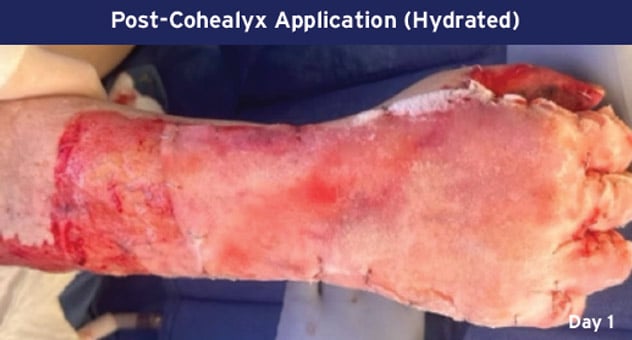
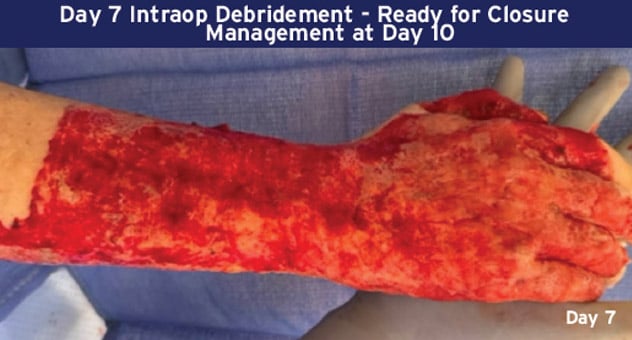
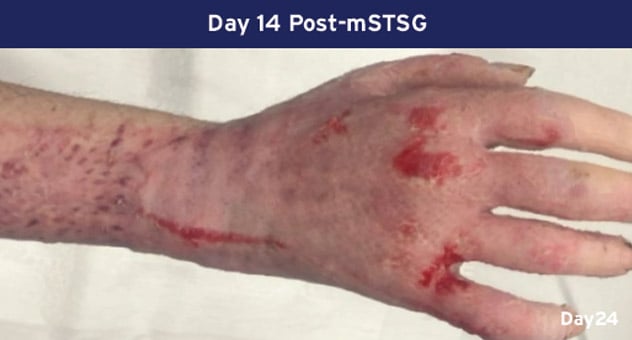
Photos Courtesy of John Loftus, MD and Ariel Rodgers, MD (The Ohio State University, Wexner Medical Center).
Case Details: 67-year-old-female with DM, previous DFUs and amputations. Left palmar hand, 32 cm2.
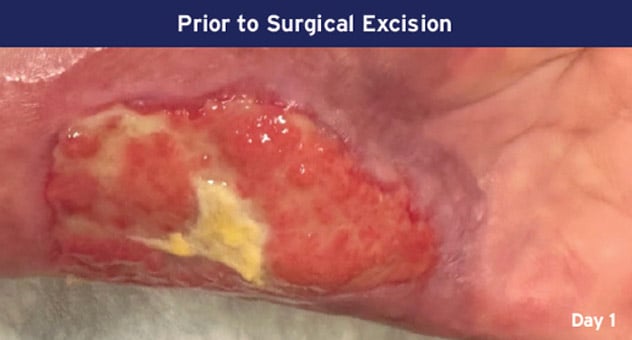
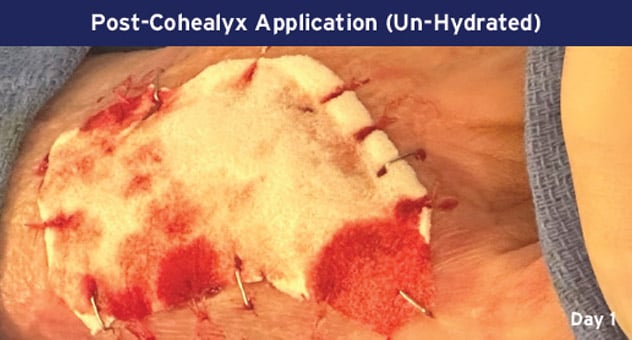
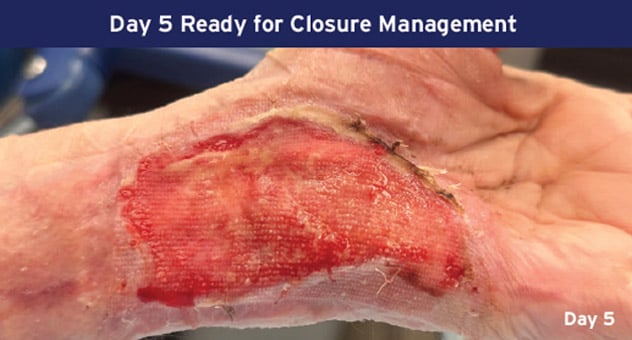
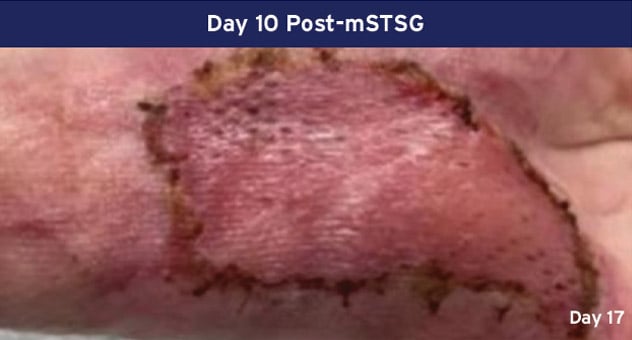
Photos Courtesy of John Loftus, MD (The Ohio State University, Wexner Medical Center).
| Model | Description | Size (cm) | cm2 | Units/Box |
| BDCDM24 | Cohealyx™ Collagen Dermal Matrix | 2 x 4 | 8 | 1 |
| BDCDM55 | Cohealyx™ Collagen Dermal Matrix | 5 x 5 | 25 | 1 |
| BDCDM1010 | Cohealyx™ Collagen Dermal Matrix | 10 x 10 | 100 | 1 |
| BDCDM1020 | Cohealyx™ Collagen Dermal Matrix | 10 x 20 | 200 | 1 |
| BDCDM2035 | Cohealyx™ Collagen Dermal Matrix | 20 x 35 | 700 | 1 |
Experience Cohealyx. Contact us to schedule an evaluation.
Call 833-462-8482 or email us at customerservice@AVITAmedical.com
mSTSG = Meshed Split Thickness Skin Graft and DM = Diabetes Mellitus, DFU = Diabetic Foot Ulcer
